Dissimilar metals
http://www.preservationscience.com/materials/metals/PGC.html
Preventing Galvanic Corrosion
By Choosing the Right Materials
Galvanic corrosion occurs when two dissimilar metals come into electrical contact with a conductive electrolyte, usually rainwater or groundwater. In this process, a metal atom is oxidized, during which it leaves its bulk metal after losing one or more electrons and is then transferred to another site. the site where the metal atoms lose electrons is called the anode, while the site where the electrons are transferred is called the cathode. (1)
The Galvanic Series is a list of metals arranges in order of their electrical potential in flowing seawater. The metals on the anodic, or active, end will corrode faster than the metals toward the cathodic, or passive, end. (2)
It is especially important to consider the potential of galvanic corrosion when choosing metal paneling, trim, and fasteners. When choosing materials, choose metals close together on the list, as metals close together generally do not have a strong effect on one another. The farther apart any two metal are on the list, the stronger the corroding effect on the more active metal. (3)
The Galvanic Series (4)
- ANODIC - active
- ---------------
- Magnesium alloys
- Zinc
- Beryllium
- Aluminum 1100, 3003, 3004, 5052, 6053
- Galvanized steel
- Cadmium
- Aluminum 2017, 2024, 2117
- Mild Steel (1018), Wrought Iron
- Cast iron, Low alloy high strength steel
- Chrome iron (active)
- Stainless steel, 430 series (active)
- Stainless steel 302, 303, 304, 321, 347, 410, 416, (active)
- Nickel (resist)
- Stainless steel 316, 317, (active)
- Carpenter 20 CB-3 stainless (active)
- Aluminum Bronze (CA 687)
- Hastelloy C (active), Inconel 625 (active), titanium (active)
- Lead-tin solders
- Lead
- Tin
- Inconel 600 (active)
- Nickel (active)
- Brasses (naval, yellow, red, admiralty)
- Copper (CA102)
- Manganese bronze, tin bronze
- Silicon bronze
- Nickel silver
- Copper-nickel alloy
- 430 stainless steel
- Nickel (passive), aluminum, bronze
- Monel 400, K500
- Silver solder
- Nickel (passive)
- Chrome iron (passive)
- 302, 303, 304, 321, 347, stainless steel (passive)
- 316, 317, stainless steel (passive)
- Carpenter 20 CB-3 stainless (passive), Incoloy 825
- Nickel-molybdeum-chromium-iron alloy (passive)
- Silver
- Titanium and titanium alloys
- Graphite
- Zirconium
- Gold
- Platinum
- ---------------
- Cathodic - passive
Minimizing Galvanic Corrosion
Use metals that are not dissimilar
Prevent dissimilar metals form becoming electrically connected by water
Keep small anodes from contacting large cathodes. The rate of corrosion depends on the surface area of the anode with respect to the cathode. The smaller the surface area of the anode relative to the surface area of the cathode, the more concentrated the flow of electrons at the anode and the faster the rate of corrosion. The larger the anode's surface area in relation to the cathode, the more spread out of the flow of electrons and the slower the rate of the anode's corrosion. (5)
The application of a protective metallic coating, known as a sacrificial coating, can provide galvanic protection to the base metal when the coating is measurably more anodic than the base metal. galvanic corrosion will take place with the anodic material when the base material is exposed. The extent to which a sacrificial coating can continue to protect the base metal is directly related to the thickness of the coating. (6)
Metallic coasting that are not sacrificial, as well as paint coatings, plastic, or other non-metallic barriers can also significantly reduce galvanic corrosion. however, when using a paint coating, it is important to realize that if the base metal becomes exposed through a small scratch in the paint, the base metal could rapidly corrode if it becomes the anode in a reaction with a nearby dissimilar metal with a large surface area. (7)
Preventing Corrosion in Fasteners
Galvanic corrosion is obviously a concern in the use of metal fasteners such as bolts, screws, and welds. Because fasteners have a much smaller surface area than the materials they fasten, fasteners that take on the role of the anode will be at risk of rapid corrosion and thus should be avoided. For example, zinc-coated fasteners should only be used to connect steel coated with aluminum, zinc, and galvalume, as these are very close on the Galvanic Series and are not generally at risk of corrosion when placed together. On the other had, zinc-coated or aluminum-coated fasteners should not be used to attach copper or stainless-steel panels.
To minimized the risk of galvanic corrosion of fasteners, match the surface metal on the fastener with that on the metal it will fasten. (8) The most desired combination of large anode with small cathode; in other words, fasteners such as bolts and screws should be made of the metal less likely to corrode, or the more cathodic.
The following chart can be used to guide the selection of fasteners based on galvanic action: (9) 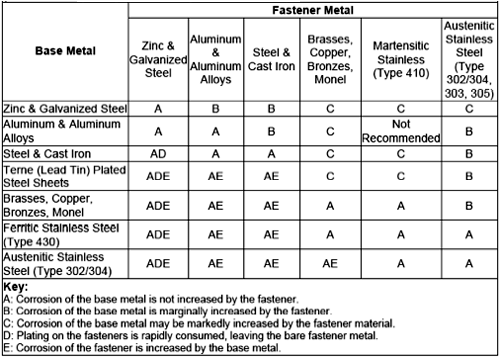
Corrosion of Panels and Trim in Contact with Treated Wood
Do not allow aluminum, aluminum-coated, and galvalume-coated panels and trim to come into direct contact with wood preservatives containing copper, mercury, or fluorides. Avoid direct contact between bare metal panels and treated lumber where condensation will frequently form on the metal surface in contact with the lumber, and where the wood treatment is more noble than the metal surface. Use an appropriate barrier to separate metal panels and treated lumber. (10)
https://www.metalsupermarkets.com/the-differences-between-304-and-316-stainless-steel/
When selecting a stainless steel that must endure corrosive environments, austenitic stainless steels are typically used. Possessing excellent mechanical properties, the high amounts of nickel and chromium in austenitic stainless steels also provide outstanding corrosion resistance. Additionally, many austenitic stainless steels are weldable and formable. Two of the more commonly used grades of austenitic stainless steel are grades 304 and 316. To help you determine which grade is right for your project, this blog will examine the difference between 304 and 316 stainless steel.
304 Stainless Steel
Grade 304 stainless steel is generally regarded as the most common austenitic stainless steel. It contains high nickel content that is typically between 8 and 10.5 percent by weight and a high amount of chromium at approximately 18 to 20 percent by weight. Other major alloying elements include manganese, silicon, and carbon. The remainder of the chemical composition is primarily iron.
The high amounts of chromium and nickel give 304 stainless steel excellent corrosion resistance. Common applications of 304 stainless steel include:
- Appliances such as refrigerators and dishwashers
- Commercial food processing equipment
- Fasteners
- Piping
- Heat exchangers
- Structures in environments that would corrode standard carbon steel.
316 Stainless Steel
Similar to 304, Grade 316 stainless steel has high amounts of chromium and nickel. 316 also contains silicon, manganese, and carbon, with the majority of the composition being iron. A major difference between 304 and 316 stainless steel is the chemical composition, with 316 containing a significant amount of molybdenum; typically 2 to 3 percent by weight vs only trace amounts found in 304. The higher molybdenum content results in grade 316 possessing increased corrosion resistance.
316 stainless steel is often considered one of the most suitable choices when selecting an austenitic stainless steel for marine applications. Other common applications of 316 stainless steel include:
- Chemical processing and storage equipment.
- Refinery equipment
- Medical devices
- Marine environments, especially those with chlorides present
Which Should You Use: Grade 304 or Grade 316?
Here are some situations where 304 stainless steel may be the better choice:
- The application requires excellent formability. The higher molybdenum content in Grade 316 can have adverse effects on formability.
- The application has cost concerns. Grade 304 is typically more affordable than Grade 316.
Here are some situations where 316 stainless steel may be the better choice:
- The environment includes a high amount of corrosive elements.
- The material will be placed underwater or be exposed to water consistently.
- In applications where greater strength and hardness are required.
When selecting a stainless steel that must endure corrosive environments, austenitic stainless steels are typically used. Possessing excellent mechanical properties, the high amounts of nickel and chromium in austenitic stainless steels also provide outstanding corrosion resistance. Additionally, many austenitic stainless steels are weldable and formable. Two of the more commonly used grades of austenitic stainless steel are grades 304 and 316. To help you determine which grade is right for your project, this blog will examine the difference between 304 and 316 stainless steel.
304 Stainless Steel
Grade 304 stainless steel is generally regarded as the most common austenitic stainless steel. It contains high nickel content that is typically between 8 and 10.5 percent by weight and a high amount of chromium at approximately 18 to 20 percent by weight. Other major alloying elements include manganese, silicon, and carbon. The remainder of the chemical composition is primarily iron.
The high amounts of chromium and nickel give 304 stainless steel excellent corrosion resistance. Common applications of 304 stainless steel include:
- Appliances such as refrigerators and dishwashers
- Commercial food processing equipment
- Fasteners
- Piping
- Heat exchangers
- Structures in environments that would corrode standard carbon steel.
316 Stainless Steel
Similar to 304, Grade 316 stainless steel has high amounts of chromium and nickel. 316 also contains silicon, manganese, and carbon, with the majority of the composition being iron. A major difference between 304 and 316 stainless steel is the chemical composition, with 316 containing a significant amount of molybdenum; typically 2 to 3 percent by weight vs only trace amounts found in 304. The higher molybdenum content results in grade 316 possessing increased corrosion resistance.
316 stainless steel is often considered one of the most suitable choices when selecting an austenitic stainless steel for marine applications. Other common applications of 316 stainless steel include:
- Chemical processing and storage equipment.
- Refinery equipment
- Medical devices
- Marine environments, especially those with chlorides present
Which Should You Use: Grade 304 or Grade 316?
Here are some situations where 304 stainless steel may be the better choice:
- The application requires excellent formability. The higher molybdenum content in Grade 316 can have adverse effects on formability.
- The application has cost concerns. Grade 304 is typically more affordable than Grade 316.
Here are some situations where 316 stainless steel may be the better choice:
- The environment includes a high amount of corrosive elements.
- The material will be placed underwater or be exposed to water consistently.
- In applications where greater strength and hardness are required.
https://en.wikipedia.org/wiki/Martensitic_stainless_steel
Martensitic stainless steel
From Wikipedia, the free encyclopedia
Jump to navigationJump to searchStainless steels may be classified by their crystalline structure into four main types: austenitic, ferritic, martensitic, and duplex.
Martensitic stainless steel is a specific type of stainless steel alloy that can be hardened and tempered through multiple ways of aging/heat treatment.[1]
Martensitic stainless steels can be high- or low-carbon steels built around the composition of iron, 12% up to 17% chromium, carbon from 0.10% (Type 410) up to 1.2% (Type 440C).[5]
- Up to about 0.4%C they are used mostly for their mechanical properties ( pumps, valves, shafts ..).
- Above, 0.4% they are used mostly for their wear resistance (cutlery surgical blades, plastic injection molds, nozzles...).
They may contain some Ni (Type 431) which allows a higher Cr and/or Mo content, thereby improving corrosion resistance and as the Carbon content is also lower, the toughness is improved. Grade EN 1.4313 (CA6NM) with a low C, 13%Cr and 4%Ni offers good mechanical properties, good castability, good weldability and good resistance to cavitation. It is used for nearly all the hydroelectric turbines in the world (including those of the huge "Three gorges " dam in China).
Additions of B, Co, Nb, Ti improve the high temperature properties, particularly creep resistance (for heat exchangers in steam turbines).
A specific grade is Type 630 (also called 17/4 PH) which is martensitic and hardens by precipitation at 475 °C.
They are hardenable by heat treatment (specifically by quenching and stress relieving, or by quenching and tempering).[6] The alloy composition, and the high cooling rate of quenching enable the formation of martensite. Tempered martensite gives steel good hardness and high toughness; used largely for medical tools (scalpels, razors and internal clamps).[7] Untempered martensite is low in toughness and therefore brittle.
The characteristic body-centered tetragonalmartensite microstructure was first observed by German microscopist Adolf Martens around 1890. In 1912, Elwood Haynes applied for a U.S. patent on a martensitic stainless steel alloy. This patent was not granted until 1919.[8]
Martensitic stainless steel can be nondestructively tested using the magnetic particle inspection method, unlike austenitic stainless steel.
Also in 1912, Harry Brearley of the Brown-Firth research laboratory in Sheffield, England, while seeking a corrosion-resistant alloy for gun barrels, discovered and subsequently industrialized a martensitic stainless steel alloy. The discovery was announced two years later in a January 1915 newspaper article in The New York Times.[9] Brearly applied for a U.S. patent during 1915. This was later marketed under the "Staybrite" brand by Firth Vickers in England and was used for the new entrance canopy for the Savoy Hotel in 1929 in London.[10]
When formability, softness, etc. are required in fabrication, steel having 0.12 per cent maximum carbon is often used in soft condition. With increasing carbon, it is possible by hardening and tempering to obtain tensile strength in the range of 600 to 900 N/mm2, combined with reasonable toughness and ductility. In this condition, these steels find many useful general applications where mild corrosion resistance is required. Also, with the higher carbon range in the hardened and lightly tempered condition, tensile strength of about 1600 N/mm2 may be developed with lowered ductility.
A common example of a Martensitic stainless steel is X46Cr13.
This post is from Adam Dickson
VANS air force discussion on Duralac
